3.2 Gases in Chemical Reactions
Keya Jani
How Gases Behave in Chemical Reactions
There are 2 key features of gases when considering chemical reactions:1
The stoichiometric factors of gases can be used to determine the molar amounts of gas when compared to other products or reactants. Recall that stoichiometric ratios should be whole-number ratios. Additionally, based on Avogadro’s law, the stoichiometric ratios can also be used to assess volume of reactants and products.
The ideal gas law can be used to determine the amount of gas to the volume, the temperature, and pressure.
Partial Pressures and Mole Fractions
Dalton’s law of partial pressures states that within a gas reaction, each component exerts a separate pressure as if it was alone in the container, and thus all the components will add up to a total pressure that is exerted on the container. Therefore, the total pressure is equal to the combined pressure of the components (see Figure 1).
- The total pressure of all the gases in the mixture is related to the total molar amount and the total volume of all the gases - think of the ideal gas law! Remember not to mix up total pressure and partial pressure when calculating the moles of a component!
- Gases can also have partial volumes, which can be calculated in a similar manner - the total volume is the sum of the partial volumes.
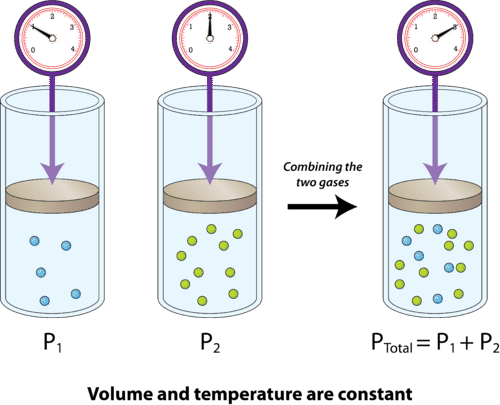
Figure 1: Dalton’s Law of Partial Pressures2
Dalton’s Law is directly applicable when collecting gases over water - since water vapor is often part of the extracted gas, there is extra pressure recorded (as the partial pressure of water adds to the partial pressure of the gas you are trying to extract). Thus, to find the real pressure of the gas, you have to subtract the vapor pressure of water at the given temperature from your measured pressure.
Mole fractions are a related concept, where instead of each component having a partial pressure or volume of the total mixture, each component has a fraction of the total moles which it contributes. This can be calculated by finding the ratio of moles, volumes, or pressures between the individual compound and the total - as summarized in the following equation:
Practice Problems
Determine the moles of product in the following reactions:
a)
b)
A 10.0L sample of air at standard temperature consists of 55% gas X, 10% gas B and the rest is gas A. How many moles of each gas are there?
a) What is the partial volume of each gas?
b) What is the partial pressure of each gas?
A 3.5 L closed beaker containing at 250 torr is connected by a valve to a 1.5 L light bulb containing at 150 torr. The valve between the two containers opens, allowing the gases to mix together. What is the mole fraction of once the gases fully mix?
References
Petrucci RH, Herring FG, Madura JD, Bissonnette C. General Chemistry: Principles And Modern Applications. 11th ed. Toronto, ON: Pearson Canada; 2017:212-217.
14.12 Dalton’s Law of Partial Pressures. CK-12 Foundation. https://flexbooks.ck12.org/cbook/ck-12-chemistry-flexbook-2.0/section/14.12/primary/lesson/daltons-law-of-partial-pressures-chem/. Published 2022. Accessed September 5, 2022.