2.1 Practice problem answers
Pranay Soma, Gurdit Sood
- There are six atoms that are not carbon or hydrogen.
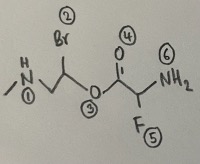
- Answer:
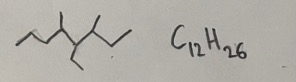
- Answer:
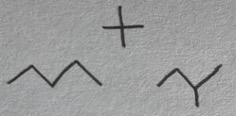
Pentane, 1-methylbutane, 2,2-dimethylpropane
4-ethyl-4,6-dimethylnonane
Answer:
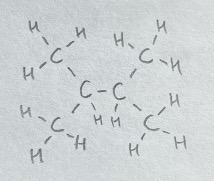
i) and ii) are constitutional isomers, as their formulas are $\ce{C4H10}$ and have different connectivities. iii) and iv) are not isomers because they are the same molecule drawn in two ways.
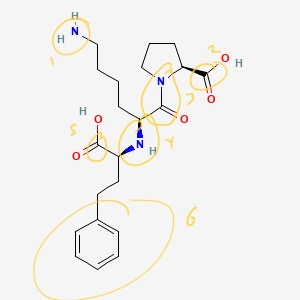
There are 6 functional groups in total: two amines, two esters, one amide, and one arene.
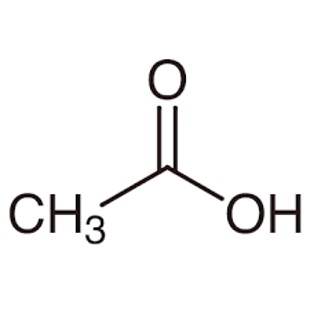
- Acetic acid is the common name for ethanoic acid. It is important to get familiar with common names since they can be tested as well.
- Alcohols contain a hydroxyl group, which allows for hydrogen bonding. This means the molecule has stronger intermolecular forces, and more energy is needed to overcome them. Therefore, it has a higher boiling point compared to hydrocarbons, which only consist of carbons and hydrogens.