2.3 Practice problem answers
Pranay Soma, Gurdit Sood
Only iii) as it is attached to 4 different substituents: methyl, ethyl, propyl, and hydrogen.
There are 10 chiral centers.
i) trans ii) neither iii) trans iv) neither
Obviously there are many possible answers, but here is an example:
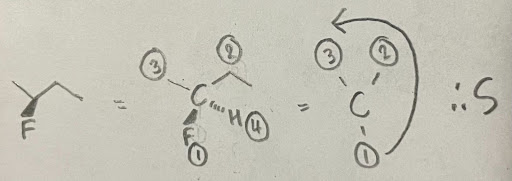
- This molecule is S configured. Be careful not to assign based on the weight of the substituents as this is a common mistake students make. Always make sure to follow the rules mentioned.
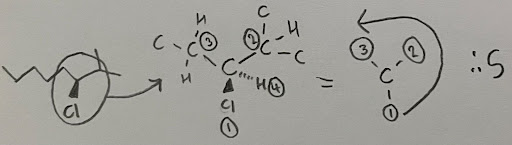
- i) R ii) R iii) S iv) R
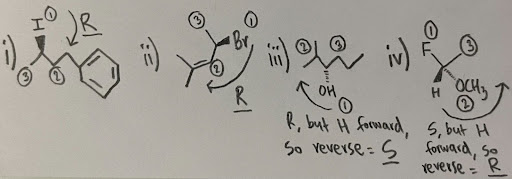
- Note: when it says “H forward, so reverse” it means that the lowest priority group is facing forward, so instead of rewriting the whole molecule, you can simply assign the configuration, and then “reverse” it to get the actual configuration. The “reversing” is the forward facing group becoming a back facing group, which is why the “configuration switch” works. This method ONLY works if the lowest priority group is forward. Please use a model kit to consolidate this concept, as this is a crucial time-saving trick.
- i) Z ii) E iii) Z iv) E
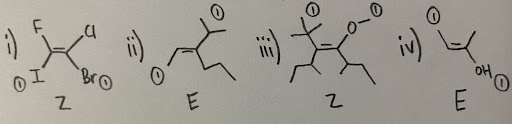
- It is important to note that in compounds with more than one chiral center, you must assign the correct configuration to the proper carbon, as free rotation does not change the stereochemistry.
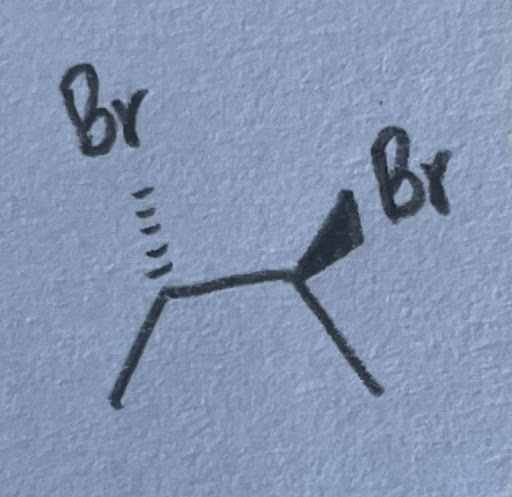
- There are 2 possible diastereomers. This is because you can change the configuration of either carbon. The molecule on the left is (2S, 3R) configured while the one on the right is (2R, 3S). Both are diastereomers of (2S, 3S)-2,3-dibromobutane.
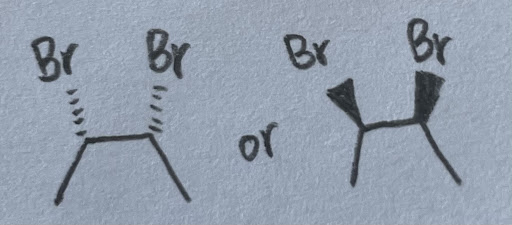
- They are a pair of enantiomers. There are two ways to go about this. One is to simply draw a dashed line (which represents a mirror) between the molecules. It is clear that they are both mirror images of each other. The longer way is to assign configurations, and it turns out that the left molecule is (2S, 3R) while the right molecule is the opposite of that (2R, 3S). This is no different from the familiar (S, S) vs. (R, R) enantiomeric pair, both chiral centers are different from each other. Same applies to (S, R) vs. (R, S), therefore these molecules are enantiomers.