2.3 Isomers, chirality, enantiomers and diasteromers
Pranay Soma, Gurdit Sood
Isomers
Isomers come in a decent amount of variety. Let’s start off with a general definition of an isomer. Isomers are molecules that have the same chemical formula, but maintain different structures and properties. Isomers can be broken down into 2 categories, constitutional isomers and stereoisomers.
Constitutional isomers are molecules that have the same molecular formula, but different connectivity. An example of constitutional isomers would be 2-methylpentane and hexane.
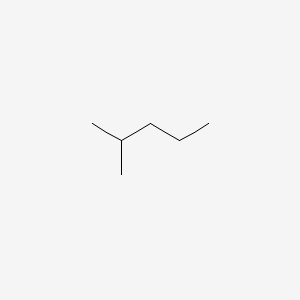
Figure 1: 2-methylpropane
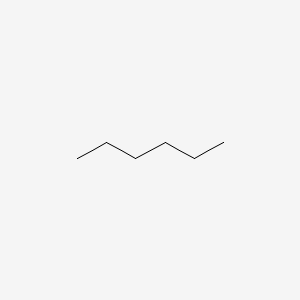
Figure 2: Hexane
Stereoisomers are a bit different. These guys have the same molecular formula, and the same connectivity, but a different arrangement of their substituents (atoms or branches bonded to a carbon) in space. Stereoisomers can be divided into 2 possible categories: enantiomers and diastereoisomers.
Enantiomers are molecules which are non-superimposable mirror images of each other, so think of your hands for example. No matter what you do, your hands cannot superimpose upon each other, and they are mirror images of each other.
Diastereoisomers are enantiomers that are not mirror images of each other, nor are they superimposable on each other4.
This flowchart summarizes the different types of isomers you should know:
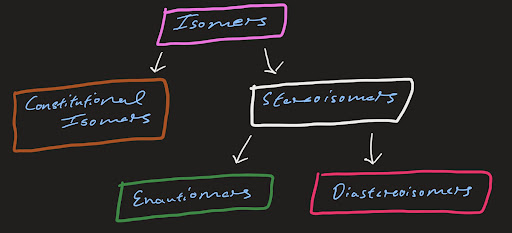
Figure 3: Isomers you should know.
Chirality
Simply defined, a chiral molecule is a molecule that has a non-superimposable mirror image. What does this mean? The term “superimposable” means that two objects can be aligned in a way that makes them completely indistinguishable. An example of this are any two objects that are identical, like a pair of dice. An example of non-superimposable images would be your hands. No matter how you turn or twist your right hand, it will never match the left hand. You can get your fingers to align perfectly when positioned in a clapping position, but one hand has the palm facing up while the other has the palm facing down. Therefore, they are not identical. This is essentially what chirality is, and the significance of the “mirror” term will be evident when talking about diastereomers.
A chiral molecule has a chiral center, which is usually a tetrahedral atom (there are a few exceptions) with four different substituents attached to it. This atom is most often a carbon, as it is easily able to form four bonds. This specification gives rise to asymmetry, which is a significant property giving chiral molecules the ability to rotate plane-polarized light. Such compounds are said to be optically active, and we can use this phenomenon to determine the presence of chiral compounds in a solution.
Naming Enantiomers
If you place a mirror in front of a chiral compound, it will have a mirror image that is not identical to it. Just like your hand, no matter how you turn or twist the molecule, you will never get it to match. They are non-superimposable images. Therefore, this pair of molecules, which exist as mirror images of each other, are known as enantiomers. The key word is “mirror” as this is what distinguishes enantiomers from diastereomers.
So now we have a pair of molecules that seem to have the same molecular formula, same connectivity of atoms, with the same molecular geometry. How do we differentiate them? By using their molecular structure. Enantiomers differ in the fact that they are positioned differently around the tetrahedral atom. Much like cis and trans alkenes, a naming system is needed to distinguish the two molecules in the pair.
This naming system is called the R, S system. This system utilizes the fact that when a certain part of the molecule is faced a certain way, a clear pattern is observed. This is achieved in a few steps, first by assigning priorities to the four different substituents in the molecule, and making the lowest priority substituent face the back. This is done to have the other three substituents facing forward, all in the same plane, so we can now determine if the numbered priorities follow a “clockwise” or “anti-clockwise” direction. If the priorities are in a clockwise order, it will be given the R configuration. If they are anti-clockwise, they will be assigned the S configuration.
The specific rules on how to assign priorities is as follows:
- The substituent with the higher atomic number takes priority over the one with a lower atomic number.
- For example, a carbon is attached to a bromine, ethyl, fluorine, and hydrogen. The bromine substituent will be given a priority of 1, the fluorine would be 2, the ethyl would be 3 (the carbon is directly attached to a carbon of the ethyl group), and the hydrogen would receive the lowest priority of 4.
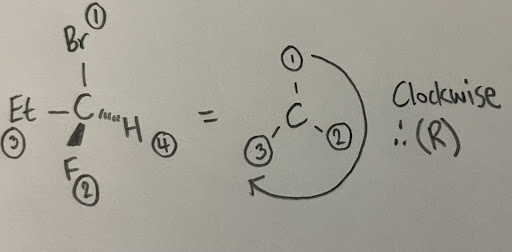
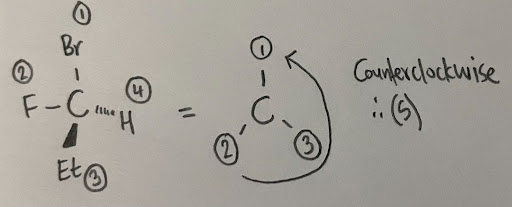
- Notice how switching the ethyl and fluorine caused the configuration to reverse. It is important to carefully assign priorities, and also make sure that the lowest priority group is always facing the back when assigning. It is also recommended to play with a model kit to see why this occurs.
If two or more substituents are the exact same atoms, then priority would depend on the subsequent atoms they are each attached to. If they continue to be the same atoms, you keep going until you find a point of difference. The atom with the higher atomic number will again be assigned higher priority.
- For example, a carbon is attached to an ethyl facing forward, hydrogen facing back, iodine, and a carbon attached to a chlorine. Since the atom is directly bonded to an iodine, two carbons, and a hydrogen, the iodine will receive a priority of 1, and the hydrogen a priority of 4. To differentiate the two alkyl chains, we must continue down until we find two different atoms. On one of the chains, we have the substituent carbon attached to two hydrogen atoms and a chlorine atom. The other chain has the substituent carbon attached to two hydrogen atoms and another carbon atom. Since the chlorine has a higher atomic number, it will receive higher priority. Thus, the chloride containing group will receive a priority of 2, and the ethyl chain will be 3.
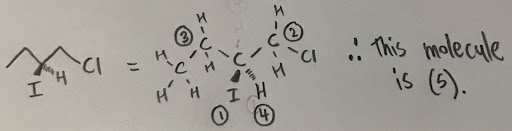
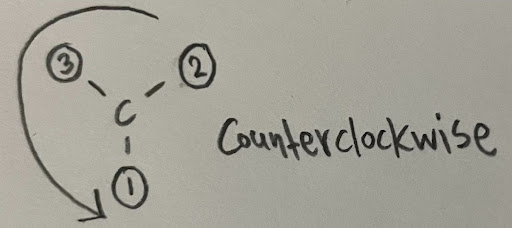
Multiple bonds are treated like “multiple single bonds”, where the atoms are replicated twice (for double bonds) or thrice (for triple bonds).
- For example, in 3-bromoprop-1-ene, the first carbon is attached to a bromine, a hydrogen, and a carbon through a double bond, while the other carbon is attached to two hydrogens and the first carbon through the double bond, In this case, you duplicate the double bond like this:
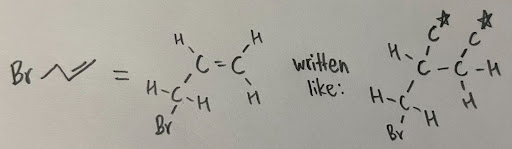
Now we can assign priorities like you normally would. It is important to note that the starred carbons are the duplicates, and they are not actually part of the molecule, it is just drawn like this to assign priorities.
Diastereomers and Naming
Diastereomers are compounds with two or more chiral centers that are not a mirror image of another stereoisomer of the same molecule. Diastereoisomers can also have the R, S system applied to them. For example:
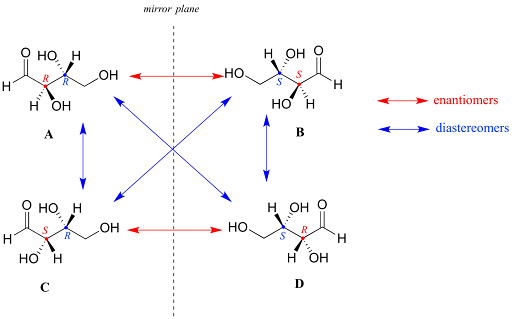
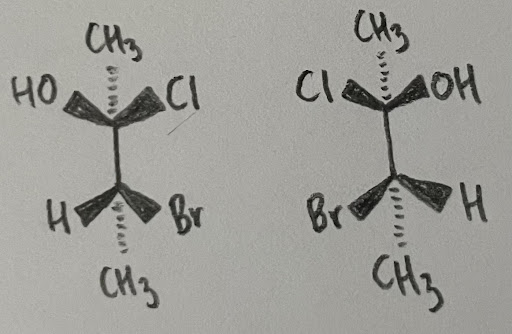
Figure 4: Enantiomeric and Diasteromeric Relationships
In this example, you can see that compounds that 1 have one chiral center different among each other are diastereomers, but 2 different chiral centers lead to an enantiomer. It is strongly recommended to play around with a model kit and try to superimpose these molecules to understand the concepts.
Cis and Trans
But what about alkenes? How can we determine their stereoisomerism? Let’s take a look at but-2-ene.

Figure 5: Depicting Cis vs. Trans Relationships using But-2-ene
When we look at the molecule on the left, we see that there are two carbons on one side, and two hydrogens on the other side of the double bond. Remember that carbon has a higher atomic number than hydrogen, so the side with the methyl group has higher priority than the hydrogens. When both carbons that are double bonded to each other have a higher priority on the same side, it is known as the “cis” isomer. When looking at trans-2-butene, we see that the higher priority is on the opposite side, hence when this happens, this is known as the “trans” isomer. Cis-trans isomers are diastereoisomers.
Another good example of diastereoisomers you should know are when they are substitutions on cyclohexanes. You should get comfortable with understanding the chair conformations of cyclohexanes as they can be tested on your exam.
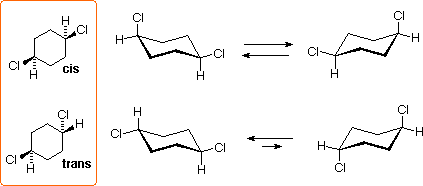
Figure 6: Cis vs. Trans with Cyclohexane Chair Conformations
When we look at the top row, notice how both chlorines point up. While one is axial up, and the other is equatorial up, both are still pointing in the same direction. This results in them gaining the cis designation, and they are represented by the same wedge as well. The bottom row has chlorines pointing in opposite directions, where both can be equatorial or axial, but they must face in opposite directions to be trans. Really in summary, understand that cis is same side, and trans is opposite side.
E and Z
What happens for alkenes that have more substituents on it other than hydrogen? Which one is cis or trans in the following?
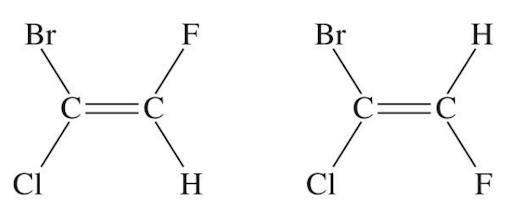
Luckily, there is a solution to this problem. Similar to the R, S system, there is E and Z for alkenes in this situation. It’s quite simple to use:
- Just like with R and S, you’re going to use the same priority rules for the substituents
- When you have 2 of the highest priority groups on the same side, that is “Z”
- If the 2 highest priority groups are on the opposite side, it is “E”
Let’s analyze an example.
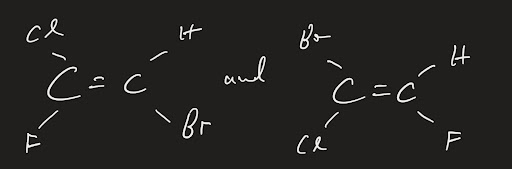
First thing to do is to recognize that the bromine has higher priority than the hydrogen, and that the chlorine has higher priority than the fluorine. As a result of this, we know that this molecule is the E isomer.

Practice Problems
- Which of the following compounds are chiral?
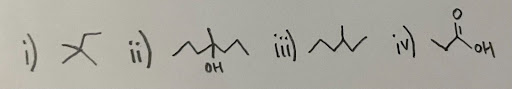
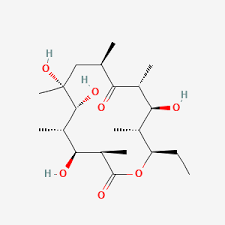
- Identify all the chiral centers in erythronolide b.
- Are the following compounds cis or trans or neither?
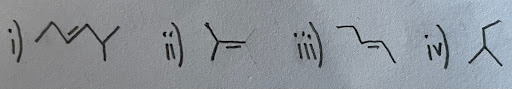
- Draw an S configured carbon with a fluorine substituent.
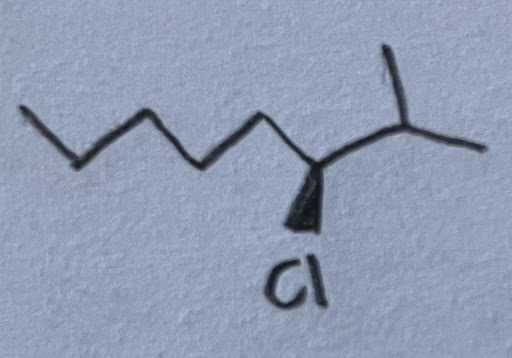
- What is the (S/R) configuration of the following molecule?
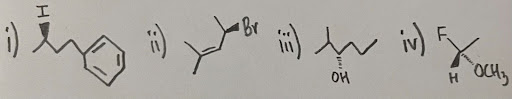
- Assign (E/Z) configurations for the following compounds:
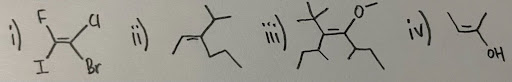
- Draw (2S, 3S)-2,3-dibromobutane.
- Draw a diastereomer of (2S,3S)-2,3-dibromobutane.
- Is this a pair of enantiomers or diastereomers?